HOCL
Info & Fact Sheet
Hypochlorous Acid
General Info.
IUPAC Name : Hypochlorous acid, chloric(I) acid, chloranol, hydroxidochlorine
Other Names : Hydrogen hypochlorite, chlorine hydroxide, electrolyzed water, electrolyzed oxidizing water, electro-activated water
CAS Number : 7790-92-3
Molar Mass : 52.46 g/mol
Molecular Formula : HOCl
Appearance : Colorless aqueous solution
Solubility in water : Soluble
Acidity : 7.53
Electrolysis in Simple Terms
Electrolysis is a process that uses electricity to cause a chemical reaction, which wouldn’t happen naturally. It’s widely used in industries to extract elements from their natural sources. The process involves passing an electric current through a substance, like saltwater, to produce different useful chemicals.
How It Works
Electricity Powers the Reaction: By using a direct electric current, electrolysis drives a chemical reaction that wouldn’t occur on its own.
Commercial Importance: Electrolysis is crucial in separating elements from their natural sources, making it an important technique in manufacturing.
Example of Electrolysis: When saltwater is subjected to electrolysis, it can produce substances like chlorine gas, bleach, hypochlorous acid, and hydrogen gas.
Historical Background
- Discovery by Michael Faraday: The laws of electrolysis were first explained by Michael Faraday in the 1830s. His work laid the foundation for the understanding and application of electrolysis in various industries.
Applications
Chemical Production: Electrolysis is used to produce a wide range of important chemicals such as chlorine gas, sodium hydroxide, and hypochlorous acid.
Environmental Uses: It can also be used to generate useful substances like ozone, which has environmental applications.
In essence, electrolysis is a powerful technique that harnesses the power of electricity to create valuable chemicals and separate elements from their natural sources.
Membrane Cell Technology
Membrane Cell Technology in Simple Terms
Membrane cell technology utilizes a special type of membrane made from a polymer that selectively allows only positive ions to pass through. This means that in the process of creating sodium hydroxide from sodium chloride solution, only the sodium ions can pass through the membrane, while the chloride ions are blocked.
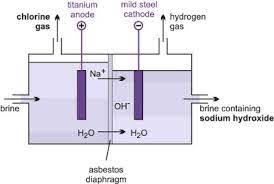
How It Works
Selective Ion Passage: The membrane only permits positive ions, such as sodium ions, to pass through, while blocking negative ions like chloride.
Preventing Contamination: This selective permeability ensures that the sodium hydroxide solution remains uncontaminated by the sodium chloride solution, as only the required ions are allowed to pass through.
Importance of Purity: The purity of the sodium chloride solution is crucial, as any other metal ions present would also pass through the membrane, leading to contamination of the sodium hydroxide solution.
In essence, membrane cell technology relies on a specialized membrane to ensure the purity of the chemical products being produced, by selectively allowing only specific ions to pass through while blocking others.
The pH
Electrolysis and pH in Simple Terms
Electrolysis is a process that uses electricity to make non-spontaneous chemical reactions happen. This technique is important in separating elements from their natural sources and is widely used in industries.

How It Works
Electricity Drives Chemical Reactions: Electrolysis uses electricity to drive chemical reactions that typically wouldn’t occur on their own.
Commercial Importance: It’s crucial in separating elements from naturally occurring sources, making it important in manufacturing.
Example of Application: Electrolysis of sodium chloride and water can produce hypochlorous acid, which is commonly used for disinfection.
Chlorine Species and pH: The distribution of chlorine species between hypochlorous acid (HOCl) and hypochlorite ion (OCl-) is determined by the pH of the solution.
- At a pH of 6.5-8.5, both HOCl and OCl- are present to some extent.
- Below a pH of 6.5, only HOCl is present, while above a pH of 8.5, only OCl- is present.
Effect on Disinfection: Hypochlorous acid is more effective for disinfection than the hypochlorite ion. Chlorination at lower pH levels is preferred for more effective disinfection, while at higher pH levels, disinfection efficiency decreases.
In essence, electrolysis is a powerful process that uses electricity to create valuable chemicals, and the type of chlorine present in the resulting solution is influenced by the pH, affecting its disinfection efficiency.
Bacteria Inactivation
Chlorine is a very viable sanitizer for inactivating microscopic organisms. A review led during the 1940s researched the inactivation levels as a component of time for E. coli, Pseudomonas aeruginosa, Salmonella typhi, and Shigella dysenteriae (Butterfield et al., 1943). Concentrate on results showed that HOCl is more successful than OCl-for inactivation of these microorganisms. These outcomes have been affirmed by a few scientists that presumed that HOCl is 70 to multiple times more viable than OCl-for inactivating microbes (Culp/Wesner/Culp, 1986). Starting around 1986, there have been many distributions affirming the prevalence of HOCl over OCl-(visit research information base ).
This greatest test has been to make hypochlorous corrosive at a close to nonpartisan pH rather than chlorine gas or hypochlorite, and to do as such in a steady structure. Hypochlorous corrosive is a meta-stable particle. It needs to return to salt water or convert to hypochlorite.
Bacteria Inactivation with Chlorine in Simple Terms
Chlorine is an effective sanitizer for killing tiny organisms like bacteria. Research conducted in the 1940s examined the levels of inactivation over time for various bacteria, and it was found that hypochlorous acid (HOCl) is more effective than the hypochlorite ion (OCl-) for killing these bacteria.
How It Works
Effectiveness of HOCl: Studies have shown that hypochlorous acid is 70 to 80 times more effective than the hypochlorite ion for killing bacteria.
pH and Stability: The challenge has been to produce hypochlorous acid at a nearly neutral pH and in a stable form. Hypochlorous acid is a meta-stable molecule, meaning it tends to revert to saltwater or convert to hypochlorite.
In essence, chlorine, specifically in the form of hypochlorous acid, is highly effective in killing bacteria, and efforts are being made to maintain its stability at a neutral pH for practical use.
In partnership with Dank Eco Solution, we are dedicated to the relentless preservation of our planet and the safeguarding of clean water for future generations. Through the innovative HOCL Technology, we strive to create a lasting legacy that will endure long after we, as parents, have moved on. Together, we are committed to ensuring a sustainable and thriving world for our children and beyond.